9. Chemical Reaction of Atmospheric Pollutants
As noted in §1, pollutants may react with each other or with the air to
form new chemicals. Some of the more important reactions for pollution
studies are now described.
9.1 Nitrogen Oxides and Ozone
The oxides of nitrogen NO (nitric oxide) and NO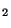 are produced during
combustion. There are two sources:
(i) From nitrogen in the air in contact with flames at a few thousand
K.
(ii) From nitrogen present within the fuel.
are produced during
combustion. There are two sources:
(i) From nitrogen in the air in contact with flames at a few thousand
K.
(ii) From nitrogen present within the fuel.
The dominant reaction producing nitrogen oxides (NO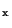 ) is
) is
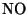 is a colourless and odourless gas. It reacts with oxygen in the air
over a few hours to produce nitrogen dioxide:
is a colourless and odourless gas. It reacts with oxygen in the air
over a few hours to produce nitrogen dioxide:
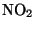 is a brown gas which is a respiratory irritant. Some of the
is a brown gas which is a respiratory irritant. Some of the 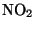 reacts with water vapour to form nitric acid:
reacts with water vapour to form nitric acid:
Another important reaction of 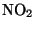 is a photochemical reaction producing
ozone,
is a photochemical reaction producing
ozone, 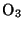 :
:
where 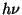 represents a photon of sunlight and
represents a photon of sunlight and 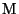 is any air
molecule (usually
is any air
molecule (usually 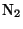 or
or 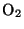 ). Ozone is the main contributor to
photochemical smog and is a strong respiratory irritant.
). Ozone is the main contributor to
photochemical smog and is a strong respiratory irritant.
9.2 Oxides of Sulphur
All fuels (e.g. oil, coal, gas, wood) contain sulphur. When these are
burnt the sulphur is mostly released as sulphur dioxide (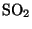 ):
):
A sequence of reactions in the atmosphere then produces sulphuric acid:
The sulphuric acid condenses onto existing particles or condenses to form new
particles. These are often captured by rain drops and fall to the ground as
acid rain.
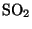 can be removed from emissions by reaction with limestone (
can be removed from emissions by reaction with limestone (
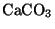 ) to form gypsum (
) to form gypsum (
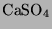 ):
):
9.3 Oxides of Carbon
Carbon dioxide (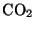 ) is a naturally occurring gas in the atmosphere.
It is absorbed by plants during photosynthesis and is therefore an essential
component of the biosphere. The amount of carbon dioxide is increasing in
the atmosphere because
(i) It is produced during the burning of all fuels
(ii) As tropical forests are cut down for timber, there are fewer
trees to absorb the
) is a naturally occurring gas in the atmosphere.
It is absorbed by plants during photosynthesis and is therefore an essential
component of the biosphere. The amount of carbon dioxide is increasing in
the atmosphere because
(i) It is produced during the burning of all fuels
(ii) As tropical forests are cut down for timber, there are fewer
trees to absorb the 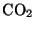 .
.
The proportion of 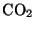 in the atmosphere has increased by about 25%
since the Industrial Revolution.
in the atmosphere has increased by about 25%
since the Industrial Revolution.
The air is quite transparent in incoming solar radiation. This heats the
surface of the Earth which in turn emits radiation at much longer
wavelengths. This long-wave radiation is strongly absorbed by 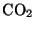 . Thus,
increasing the proportion of
. Thus,
increasing the proportion of 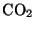 has the potential to increase the
temperature of the atmosphere and oceans (global warming). It is thought that
doubling the amount of
has the potential to increase the
temperature of the atmosphere and oceans (global warming). It is thought that
doubling the amount of 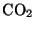 over the next century would result in a
temperature rise of 0.5-5
over the next century would result in a
temperature rise of 0.5-5 C. There are great uncertainties,
particularly connected with the response of the biosphere and absorption of
C. There are great uncertainties,
particularly connected with the response of the biosphere and absorption of
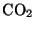 by the oceans. The main consequences of global warming would be
(i) changes in climate patterns (with desertification in places)
(ii) rising sea levels due to melting of land ice and thermal
expansion of the sea.
by the oceans. The main consequences of global warming would be
(i) changes in climate patterns (with desertification in places)
(ii) rising sea levels due to melting of land ice and thermal
expansion of the sea.
Burning of fossil fuels also produces carbon monoxide (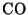 ). This is very
poisonous. It is produced in equilibrium with
). This is very
poisonous. It is produced in equilibrium with 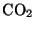 :
:
At high temperatures the equilibrium moves more to the right in the above
equation.
9.4 CFCs
CFCs (chloro-fluoro-carbons) are compounds containing chlorine, fluorine
and carbon. They are very inert, non-toxic, non-inflammable, invisible and
odourless. They have been used as refrigerants and as propellants in aerosol
cans. The problem with CFCs is that when they reach the stratosphere (10-50
km) they can release chlorine which reacts to destroy naturally occurring
ozone. For example
Stratospheric ozone is responsible for absorbing much of the ultra-violet
part of the incident solar radiation. Loss of ozone results in increased UV
radiation reaching the surface. This is harmful to humans and animals and
possibly to plants. CFCs are rapidly being replaced by other chemicals as a
result of international agreements. However, their residence time in the
atmosphere is tens to hundreds of years, so the effects of 20 century CFC production will continue for a considerable time.
century CFC production will continue for a considerable time.
back to syllabus
![]() ) is
) is
![]() can be removed from emissions by reaction with limestone (
can be removed from emissions by reaction with limestone (
![]() ) to form gypsum (
) to form gypsum (
![]() ):
):
![]() in the atmosphere has increased by about 25%
since the Industrial Revolution.
in the atmosphere has increased by about 25%
since the Industrial Revolution.
![]() . Thus,
increasing the proportion of
. Thus,
increasing the proportion of ![]() has the potential to increase the
temperature of the atmosphere and oceans (global warming). It is thought that
doubling the amount of
has the potential to increase the
temperature of the atmosphere and oceans (global warming). It is thought that
doubling the amount of ![]() over the next century would result in a
temperature rise of 0.5-5
over the next century would result in a
temperature rise of 0.5-5![]() C. There are great uncertainties,
particularly connected with the response of the biosphere and absorption of
C. There are great uncertainties,
particularly connected with the response of the biosphere and absorption of
![]() by the oceans. The main consequences of global warming would be
(i) changes in climate patterns (with desertification in places)
(ii) rising sea levels due to melting of land ice and thermal
expansion of the sea.
by the oceans. The main consequences of global warming would be
(i) changes in climate patterns (with desertification in places)
(ii) rising sea levels due to melting of land ice and thermal
expansion of the sea.
![]() ). This is very
poisonous. It is produced in equilibrium with
). This is very
poisonous. It is produced in equilibrium with ![]() :
: