7. Deposition
For some pollutants, what matters is not only the concentration in the
air but also the amount which settles on the ground. For example, dust
particles from a quarry may cause a nuisance down wind. Sulphuric acid which
is created from power station emissions causes acidification of soil and can
be harmful to plants and animals (e.g. destruction of forests and fish in lakes
by acid rain). Methods for calculating the rate of deposition are needed.
We will not go into details here. However, some important factors are:
(i) Settling rate of particles. This is strongly dependent on the
particle size and hence a knowledge of particle size distributions is
important.
(ii) Dry deposition by diffusion. The flux of a pollutant with
concentration 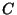 kgm
kgm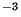 through the ground is
through the ground is
Note that in the image model for plumes reflecting from the ground,
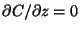 at the ground (by symmetry) and hence the
deposition rate is zero.
(iii) Wet deposition. Important processes are washout (collection
of pollutant by rain drops as they fall) and rainout (collection of pollutants
by cloud droplets which subsequently form rain drops). Knowledge of
precipitation rates is clearly essential.
at the ground (by symmetry) and hence the
deposition rate is zero.
(iii) Wet deposition. Important processes are washout (collection
of pollutant by rain drops as they fall) and rainout (collection of pollutants
by cloud droplets which subsequently form rain drops). Knowledge of
precipitation rates is clearly essential.
back to syllabus